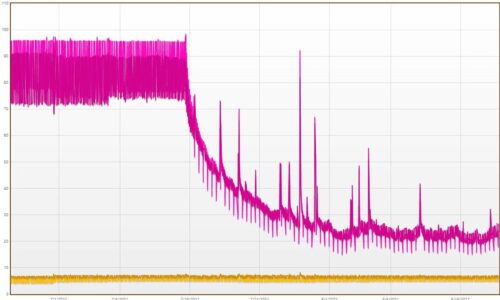
Taking control of cold chamber humidity
In any standard refrigerated chamber, the average humidity will read between 90 – 100% relative humidity (RH). When RH can rise to 90 – 100% in a cold chamber, the formation of condensation is pretty much inevitable so how can you take control of cold chamber humidity?











